Attempted
Correct
UnAttempted
Wrong
Read the passage given below and answer the question that follow :J.W. Gibbs and H.Von Helmoltz had given two equations which are known as Gibbs-Helmholtz equation. One equation can be expressed in terms of change in free energy (ΔG) and enthalpy(ΔH) while other can be expressed in terms of change in internal energy (ΔE) and work function (ΔW)
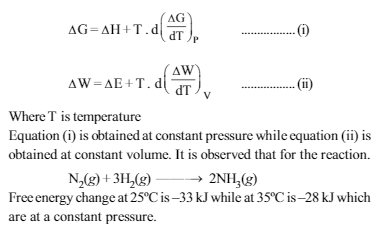
What would be the difference between enthalpy change at 25ºC and 35º C for a given reaction ?



The standard enthalpy of formation of NH3 is – 46.0 kJ mol-1.If the enthalpy of formation of H2 from its atoms is– 436 kJ mol-1 and that of N2 is – 712 kJ mol-1, the average bond enthalpy of N – H bond in NH3 is

Identify the correct statement regarding a spontaneous process
Spontaneity of reaction depends on tendency to acquire minimum energy state and maximum randomness. For a spontaneous process in an isolated system the change in entropy is positive.
Equal volumes of two monoatomic gases, A and B, at same temperature and pressure are mixed. The ratio of specific heats (Cp/Cv) of the mixture will be :

The enthalpy of fusion of water is 1.435 kcal/mol.The molar entropy change for the melting of ice at 0°C is :

Enthalpy change for the reaction, 4H(g) ⟶ 2H2(g) is – 869.6 kJ.
The dissociation energy of H–H bond is :

If the enthalpy change for the transition of liquid water to steam is 30 kJ mol-1 at 27ºC, the entropy change for the process would be :

Three moles of an ideal gas expanded spontaneously into vacuum. The work done will be :
Ideal gas during spontaneous expansion into vacuum does not do any external work.

