Attempted
Correct
UnAttempted
Wrong
DIRECTIONS : These are Assertion-Reason type questions. Each of these question contains two statements:Statement-1 (Assertion) and Statement-2 (Reason). Answer these question from the following four options.
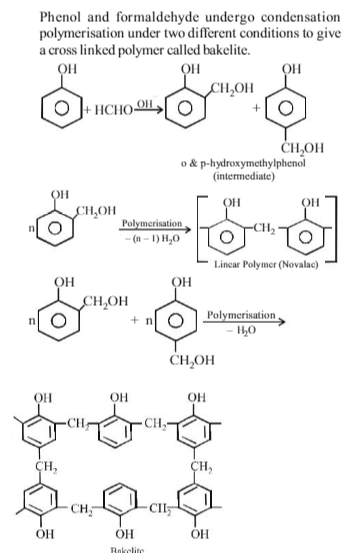
Statement-1 : Bakelite is a thermosetting polymer.
Statement-2 : Bakelite can be melted again and again without any change.
Bakelite can be heated only once.
DIRECTIONS : These are Assertion-Reason type questions. Each of these question contains two statements:Statement-1 (Assertion) and Statement-2 (Reason). Answer these question from the following four options.
Statement-1 :In vulcanisation of rubber, sulphur cross links are introduced.
Statement-2 : Vulcanisation is a free radical initiated chain reaction.
Vulcanisation is a process of treating natural rubber with sulphur or some compounds of sulphur under heat so as to modify its properties. This cross-linking give mechanical strength to the rubber.
Among cellulose, poly (vinyl chloride), nylon and natural rubber, the polymer in which the intermolecular force of attraction is weakest is
Nylon and cellulose, both have intermolecular hydrogen bonding, polyvinyl chloride has dipole-dipole interactions, while natural rubber has van der Waal forces which are weakest.
The polymer containing strong intermolecular forces e.g.hydrogen bonding, is
Nylon 6, 6 has amide linkage capable of forming hydrogen bonding.
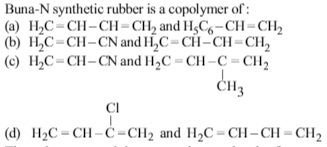
Buna –N is a copolymer of butadiene(CH2 = CH–CH = CH2) and acrylonitrile (CH2 = CHCN).
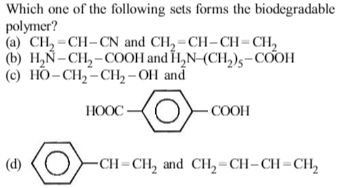
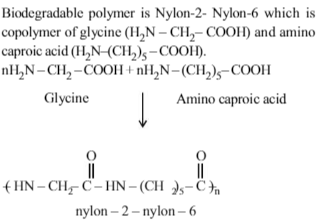
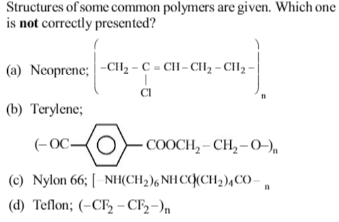
Bakelite is obtained from phenol by reacting with
Which of the following statements is false?
Nylon(polyamides) are fibres.
Which one of the following is not a condensation polymer ?