In Goldschmidt alumino thermic process which of the following reducing agents is used :
-
Solution
Reduction by powdered aluminium is known as Gold-Schmidt aluminothermic process. This process is employed in cases where metals have very high m.p.and are to be extracted from their oxides.
Consider the following statements –
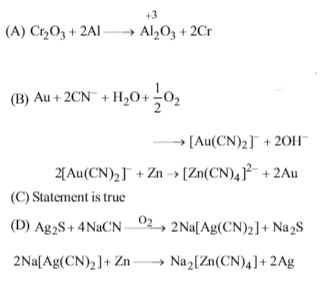
(A)In the Aluminothermite process, aluminium acts as reducing agent.
(B)The process of extraction of gold involves the formation of [Au(CN)2]– and [Zn(CN)4]2-.
(C)In the extractive metallurgy of zinc, partial fusion of ZnO with coke is called sintering and reduction of ore to the molten metal is called smelting.
(D)Extractive metallurgy of silver from its ore argentine involves complex formation and displacement by more electropositive metal.Choose the correct options –
-
Solution

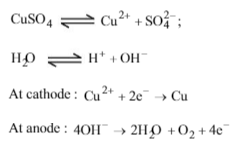
When CuSO4 is electrolysed using platinum electrodes,
-
Solution

Calcination is the process in which :
-
Solution
Calcination is a process of heating a substance to a high temperature but below the melting or fusion point,causing loss of moisture, reduction or oxidation and dissociation into simpler substances.
While extracting an element from its ore, the ore is grounded and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is
-
Solution
Cyanide process is used in the metallurgyof Ag
2Ag2S + 8NaCN + O2 2H2O ⟶ 4Na[Ag(CN)2] + 4NaOH + 2S
2Na[Ag(CN)2] + Zn ⟶ Na2[Zn(CN)4]2Ag↓
According to Ellingham diagram, the oxidation reaction of carbon to carbon monoxide may be used to reduce which one of the following oxides at the lowest temperature ?
-
Solution
In the graph of ∆rG° vs T for formation of oxides, the Cu2O line is almost at the top. So, it is quite easy to reduce oxide ores of copper directly to the metal by heating with coke both the lines of C, CO and C, CO2 are at much lower temperature (500 - 600 K).
Cu2O + C ⟶ 2Cu + CO
In electro-refining of metal the impure metal is used to make the anode and a strip of pure metal as the cathode, during the electrolysis of an aqueous solution of a complex metal salt. This method cannot be used for refining of
-
Solution
Na reacts vigorously with water (exothermic process ).
Refractory metals are used in construction of furnaces because
-
Solution
Refractory metals are used in the construction of furnaces because they can withstand high temperature e.g. silica, flint, lime, etc.Option (a) is correct
Method used for obtaining highly pure silicon which is used as a semiconductor material, is
-
Solution
Si obtained by reduction of SiCl4 with H2 is further purified by zone refining method to get Si of very high purity. Silicon is purified by zone-refining process because the impurities present in it are more soluble in the liquid phase than in the solid phase.
The elements present in the core of earth are collectively known as