DIRECTIONS: These are Assertion-Reason type questions. Question contains two statements:Statement-1 (Assertion) and Statement-2 (Reason). Answer these question from the following four options.
Statement-1 Levigation is used for the separation of oxide ores from impurities.
Statement-2 Ore particles are removed by washing in a current of water.
-
Solution
Statement-1 is true but Statement-2 is false.Oxide ores being heavier than the earthy or rocky gangue particles, settle down while lighter impurities are washed away.
DIRECTIONS: These are Assertion-Reason type questions. Question contains two statements:Statement-1 (Assertion) and Statement-2 (Reason). Answer these question from the following four options.
Statement-1 Lead, tin and bismuth are purified by liquation method.
Statement-2 Lead, tin and bismuth have low m.p. as compared to impurities
In the extraction of copper from its sulphide ore, the metal is finally obtained by the reduction of cuprous oxide with :
-
Solution
Cuprous oxide formed during roasting of cuprous sulphide is mixed with few amount of cuprous sulphide and heated in a reverberatory furnace to get metallic copper
2Cu2O + Cu2S ⟶ 6Cu + SO4
Which one of the following is a mineral of iron ?
-
Solution
Fe3O4 – Magnetite
CuCO3· Cu(OH)2– Malachite
Pyrolusite – MnO2 and Cassiterite – SnO2.
Aluminium is extracted from alumina (Al2O3) by electrolysis of a molten mixture of :
-
Solution
Fused alumina (Al2O3) is a bad conductor of electricity.Therefore, cryolite (Na3AlF6) and fluors par (CaF2) are added to purified alumina which not only make alumina a good conductor of electricity but also reduce the melting point of the mixture to around 1140 K.
The following reactions take place in the blast furnace in the preparation of impure iron. Identify the reaction pertaining to the formation of the slag.
-
Solution
In blast furnace at about 1270 K, calcium carbonate is almost completely decomposed to give CaO which acts as a flux and combines with SiO2 present as impurity(gangue) in the ore to form calcium silicate (fusible slag)
CaO(s) (basic flux) +SiO2(s)(acidic flux) ⟶ CaSiO3(s)(slag)
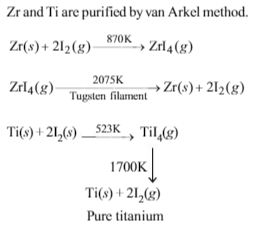
Which of the following pairs of metals is purified by van Arkel method ?
-
Solution

Which of the following elements is present as the impurity to the maximum extent in the pig iron ?
-
Solution
Pig iron or cast iron contains 3 –5% carbon and varying amounts of Mn,Si, P and S which makes the iron hard and brittle.
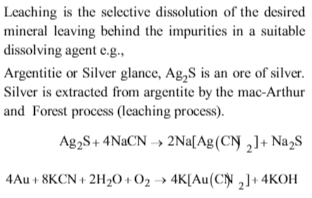
Sulphide ores of metals are usually concentrated by froth flotation process. Which one of the following sulphide ores offer an exception and is concentrated by chemical leaching?
-
Solution

Which of the following statements, about the advantage of roasting of sulphide ore before reduction is not true?
-
Solution
The sulphide ore is roasted to oxide before reduction because the of \(\Delta G_{f}^{°}\) of most of the sulphides are greater than those of CS2 and H2S, therefore neither C nor H can reduce metal sulphide to metal. Further, the standard free energies of formation of oxide are much less than those of SO2. Hence oxidation of metal sulphides to metal oxide is thermodynamically favourable.