Attempted
Correct
UnAttempted
Wrong
If methyl is alkyl group, then which order of basicity is correct
R2NH > RNH2 > R3N > NH3
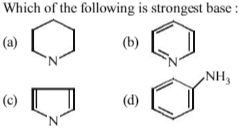
In (b), (c) and (d) lone pair of nitrogen is linked with sp2 hybridised carbon which is acidic in nature therefore it attracts the electron pair towards itself. In (a) lone pair of nitrogen is free because it is attached with carbon which is sp3 hybridised. So it is most basic.
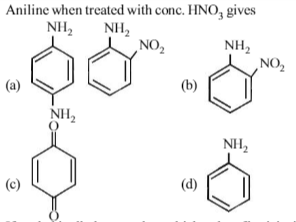
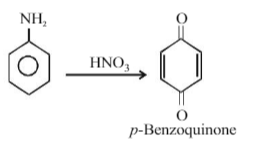
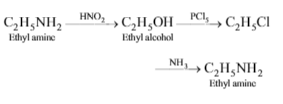
The end product of the reactions is
\(C_{2}H_{5}NH_{2} \overset{HNO_{2}}{\rightarrow} A \overset{PCl_{5}}{\rightarrow} B \overset{H.NH_{2}} {\rightarrow} C\)
The structural formula of methyl aminomethane is
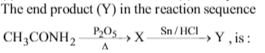

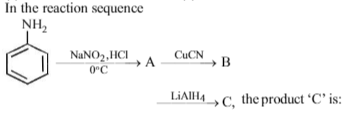
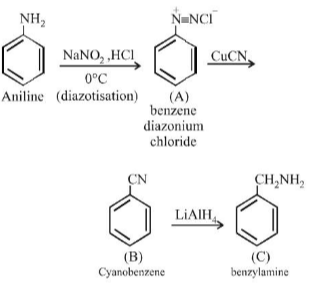
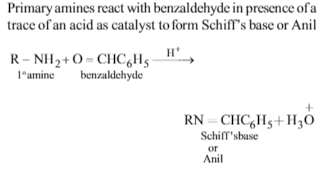
Primary amines react with benzaldehyde to form:
Nitrosoamines (R2N – N = O) are soluble in water. On heating them with concentrated H2SO4, they give secondary amines.This reaction is called
The given reaction is known as Liebermann Nitroso reaction.
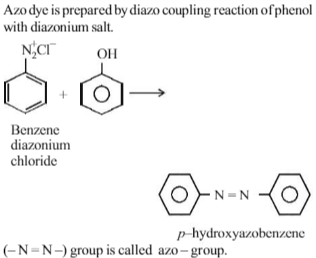
Azo dye is prepared by the coupling of phenol and