Attempted
Correct
UnAttempted
Wrong
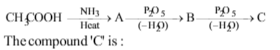

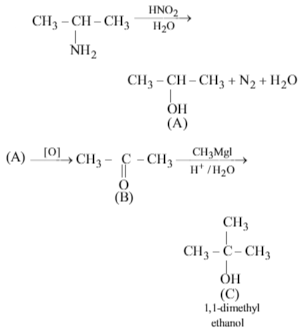
An organic amino compound reacts with aqueous nitrous acid at low temperature to produce an oily nitrosoamine. The compound is
Since the organic amino compound on reaction with nitrous acid at low temperature produces an oily nitrosoamine so the organic amino compound is a secondary aliphatic amines.
Nitrobenzene and hydrogen in presence of zinc combines to form :
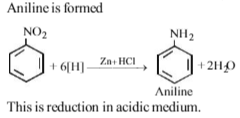
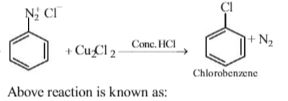
The given reaction is known as Sandmeyer ’s reaction.
In the reaction,
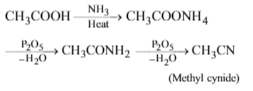
\(RNH_{2} \overset{HNO_{2}}{\rightarrow} A + B + C\)↑; C is
![]()
High basicity of Me2NH relative to Me3N is attributed to :
Secondary amines are more basic than tertiary amines due to stabilisation of 2° amine by hydrogen bonding with solvent molecule.
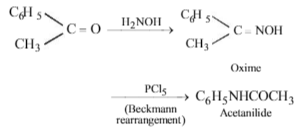
The conversion of acetophenone to acetanilide is best accomplished by using :
p-Chloroaniline and anilinium hydrogen chloride can be distinguished by:
p-Chloroaniline and anilinium hydrogen chloride can be distinguished by AgNO3. Anilinium hydrogen chloride will give white ppt of AgCl on reaction with AgNO3 but p-chloronoaniline will not react with it because Cl is directly attached to benzene nucleus.
In the diazotization of arylamines with sodium nitrite and hydrochloric acid, an excess of hydrochloric acid is used primarily to
Excess of HCl is used to convert free aniline to aniline hydrochloride otherwise free aniline would undergo coupling reaction with benzenediazonium chloride.