Attempted
Correct
UnAttempted
Wrong
Among the following amines, which one has the highest pKb value in aqueous solution?
Weak base has high pkb value. Benzenamine (aniline)is the weakest base among the given amines. Therefore it has highest pkb value.
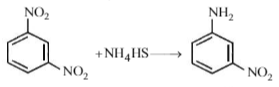
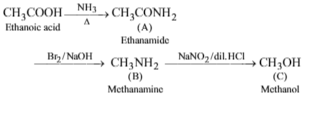
Ethanoic acid on heating with ammonia forms compound A which on treatment with bromine and sodium hydroxide gives compound B. Compound B on treatment with NaNO2/dil.HCl gives compound C. The compounds A, Band C respectively are
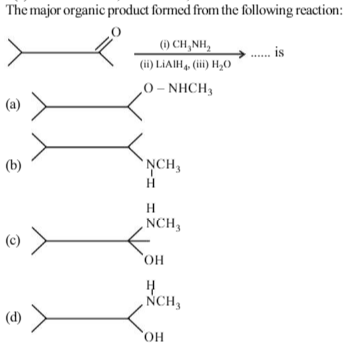
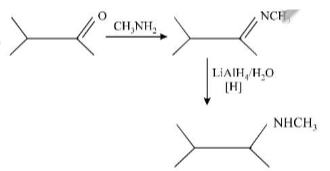
Choose the incorrect statement
