Attempted
Correct
UnAttempted
Wrong
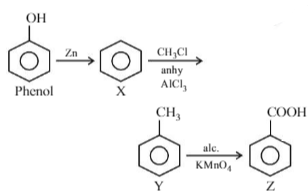
The correct order of acid strength of the following compounds :
(A)Phenol
(B)p–Cresol
(C)m–Nitrophenol
(D)p–Nitrophenol
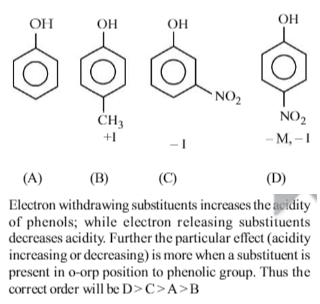
From amongst the following alcohols the one that would react fastest with conc. HCl and anhydrous ZnCl2, is
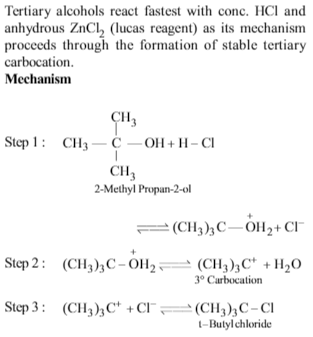
The major product obtained on interaction of phenol with sodiumhydroxide and carbon dioxide is
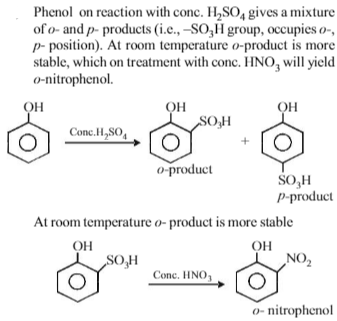
Phenol, when it first reacts with concentrated sulphuric acid and then with concentrated nitric acid, gives
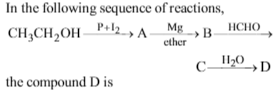
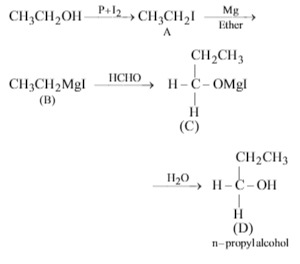
Which of the following compounds can be used as antifreezein automobile radiators ?
Glycol is used as an antifreeze in automobiles.
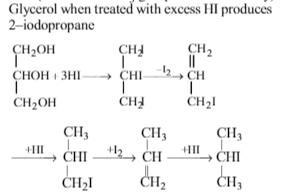
When glycerol is treated with excess of HI, it produces:
Among the following four compounds
(i)phenol
(ii)methylphenol
(iii)meta-nitrophenol
(iv)para-nitrophenol the acidity order is :
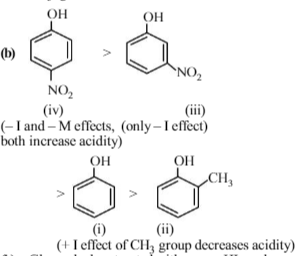
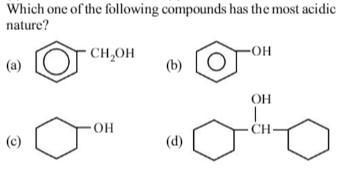
Phenol is most acidic because its conjugate base is stabilised due to resonance, while the rest three compounds are alcohols, hence, their corrosponding conjugate bases do not exhibit resonance.