Attempted
Correct
UnAttempted
Wrong
Ether which is liquid at room temperature is
CH3OCH3 and C2H5OCH3 are gases while C2H5OC2H5(b. p. 308 K) is low boiling liquid.
Which of the following product is formed, when ether is exposed to air ?
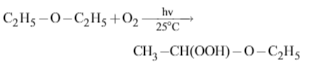
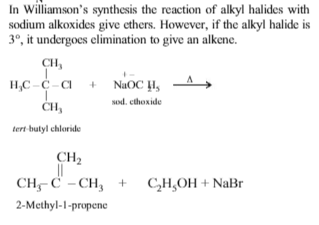
The major product of the reaction between tert-butyl chloride and sodium ethoxide is
The reaction of sodium ethoxide with ethyl iodide to form diethyl ether is termed
Reaction of sodium ethoxide with ethyl iodide to produce diethyl ether is known as Williamson synthesis.It is anucleophilic substitution reaction and proceeds via SN2 mechanism.
A fruity smell is produced by the reaction of C2H5OH with

Formation of diethyl ether from ethanol is based on a
Dehydration of alcohols gives ethers.


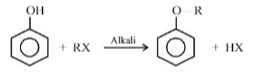
In Williamson’s synthesis, ethoxyethane is prepared by
Williamson’s synthesis -
CH3 - CH2 - ONa + Cl - CH2 - CH3 ⟶ CH3 - CH2 - O - CH2 - CH3
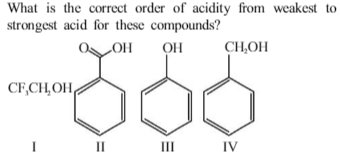
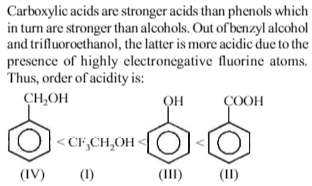
In the reaction \(Ar + OH + RX \overset{pyridine}{\rightarrow} A\) ,A is