Attempted
Correct
UnAttempted
Wrong



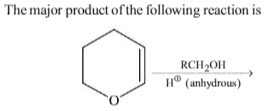
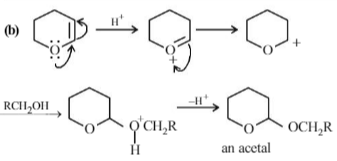
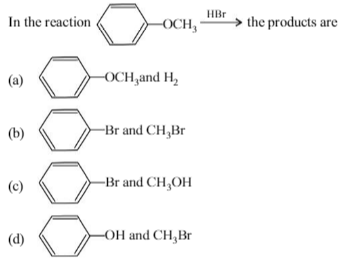
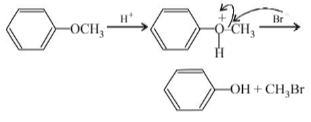
Which of the following reagents may be used to distinguish between phenol and benzoic acid?
Phenol gives a violet colour with neutral ferric chloride solution whereas benzoic acid does not give this test.
DIRECTIONS : These are Assertion-Reason type questions.Question contains two statements:Statement-1 (Assertion) and Statement-2 (Reason). Answer these question from the following four options.
Statement-1 :Lucas reagent is a mixture of anhydrous ZnCl2 and concentrate HCl
Statement-2 :Primary alcohol produce ppt. with Lucas reagents
Lucas reagent is a mixture of anhydrous ZnCl2 and conc. HCl. It is used for the distinction of monohydric alcohol. Tertiary alcohols on addition produce a precipitate immediately while secondary alcohols produce ppt. after 5 minutes. Primary alcohols do not produce any precipitate. Therefore, statement-1 is true but statement-2 is false.
DIRECTIONS : These are Assertion-Reason type questions.Question contains two statements:Statement-1 (Assertion) and Statement-2 (Reason). Answer these question from the following four options.
Statement-1 :Phenol undergo Kolbe reaction, ethanol does not.
Statement-2 :Phenoxide ion is more basic than ethoxide ion.
It is correct that sodium phenoxide (sodium salt of phenol) and CO2 on heating form sodium salicylate.This is known as Kolbe’s reaction. Ethanol does not respond to this reaction. Therefore, statement-1 is true.But the statement-2 that phenoxide ion is more basic than ethoxide ion is not correct.
DIRECTIONS : These are Assertion-Reason type questions.Question contains two statements:Statement-1 (Assertion) and Statement-2 (Reason). Answer these question from the following four options.
Statement-1 :A triester of glycerol and palmitic acid on boiling with aqueous NaOH gives a solid cake having soapy touch
Statement-2 :Free glycerol is liberated which is a greasy solid
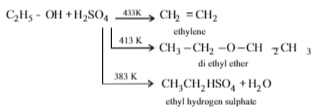
Consider the following reaction :C2H5OH + H2SO4 ⟶ Product
Among the following, which one cannot be formed as a product under any conditions ?