The role of calcination in metallurgical operations is

-
Solution

On mixing ethyl acetate with aqueous sodium chloride, the composition of the resultant solution is
-
Solution
There is no reaction hence the resultant mixture contains CH3 COOC2H5+ NaCl
Which of the following represents correct set of the four quantum numbers for an electron in a 4d subshell ?
-
Solution

The mass of a molecule of water is
-
Solution
Mass of one molecule of Water
= \(\frac{18}{6.023 \times 10^{23}}\)
=3 × 10-23g = 3 × 10-26kg
The combination of three 2p orbitals of one atom and three 2p orbitals of another atom can give rise to :
-
Solution
Three 2p orbitals of one atom and three 2p orbitals of another atom give rise to four(π 2p) and two (σ 2p) molecular orbitals.
Among 2px, 2py and 2pz orbitals of two atoms 2pz combines with 2pz along z-aixs give rise to two π 2p molecular orbitals, while 2px combines with 2px and 2py combines with 2py give rise to two π 2px and two σ 2py molecular orbitals.
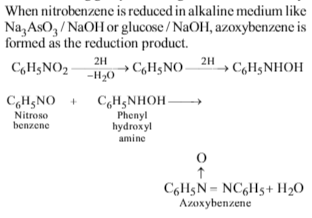
Nitrobenzene is reduced to azoxybenzene using
-
Solution

If xis amount of adsorbate and m is amount of adsorbent,which of the following relations is not related to adsorption process ?
Lanthanum is grouped with f-block elements because
Identify the incorrect statement among the following:
-
Solution
4f orbital is nearer to nucleus as compared to 5f orbital therefore, shielding of 4 f is more than 5f.
These are Assertion-Reason type questions. Each of these question contains two statements:Statement-1 (Assertion) and Statement-2 (Reason). Answer these questions from the following four options.
Statement-1 :Acetic acid does not undergo haloform reaction.
Statement-2 :Acetic acid has no alpha hydrogens.
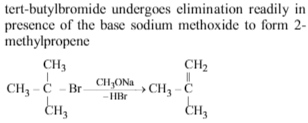
What are the starting materials to get 2-methylpropene as the major product ?
-
Solution

The melting point of lithium (181°C) is just double the melting point of sodium (98°C) because –
-
Solution
The atom becomes larger on descending the group, so the bonds becomes weaker (metallic bond), the cohesive force/energy decreases and accordingly melting point also decreases.
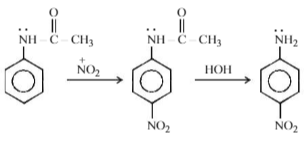
Acetanilide on nitration followed by alkaline hydrolysis mainly gives –
-
Solution

Dehydration of alcohol is an example of
-
Solution

The molecular weight of O2 and SO2 are 32 and 64 respectively. At 15°C and 150 mm Hg pressure, one litre of O2 contains ‘N’ molecules. The number of molecules in two litres of SO2 under the same conditions of temperature and pressure will be:
-
Solution
According to Avogadro's law "equal volumes of all gases contain equal number of molecules under similar conditions of temperature and pressure".
Thus if 1 L of one gas contains N molecules, 2 L of any other gas under the same conditions of temperature and pressure will contain 2N molecules.
Which of the following substances acts as an oxidising as well as a reducing agent?
-
Solution
In Na2O, SnCl2 and Na2O2 central atom is either in lowest or highest oxidation state, so it can function either as an oxidising or a reducing agent but not both.However, the oxidation state of N in NaNO2 is +3 which lies between its highest(+5) and lowest (–3) values.
For advertisement, the coloured discharge tubes contain :
A mixture of components A and B will show –ve deviation when
-
Solution
A solution containing A and B components shows negative deviation when A–A and B–B interactions are weaker than that of A–B interactions. For such solutions.ΔH = –ve and ΔV = –ve.
The oxide of lead used in lead accumulators is :
-
Solution
PbO2 is a strong oxidising agent and is produced in situ in lead storage batteries. The anode is oxidized to PbO2 and cathode is reduced to spongy Pb.