Attempted
Correct
UnAttempted
Wrong
Read the passage given below and answer the question that follow :J.W. Gibbs and H.Von Helmoltz had given two equations which are known as Gibbs-Helmholtz equation. One equation can be expressed in terms of change in free energy (ΔG) and enthalpy(ΔH) while other can be expressed in terms of change in internal energy (ΔE) and work function (ΔW)
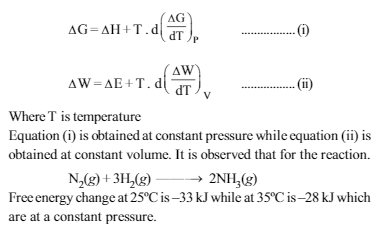
What would be the free energy change at 30º C ?

In conversion of lime-stone to lime,
CaCO3(s) ⟶ CaO(s) + CO2(g) the values of ΔH° and ΔS° are + 179.1 kJ mol-1 and 160.2 J/K respectively at 298 K and 1 bar. Assuming that ΔH° and ΔS° do not change with temperature, temperature above which conversion of limestone to lime will be spontaneous is

Standard enthalpy of vapourisation ΔvapH° for water at 100°C is 40.66 kJ mol-1. The internal energy of vaporisation of water at 100°C (in kJ mol-1) is : (Assume water vapour to behave like an ideal gas).




For B + D ⟶ E + 2C, ΔH will be :







The values of ΔH and ΔS for the reaction,C(graphite) + CO2 (g) ⟶ 2CO(g) are 170 kJ and 170 JK-1,respectively. This reaction will be spontaneous at
ΔG = ΔH – TΔS
At equilibrium, ΔG = 0
⥤ 0 = (170 × 103J) – T (170 JK-1)
⥤ T = 1000 K
For spontaneity, ΔG is – ve, which is possible only if T > 1000 K.
Bond dissociation enthalpy of H2, Cl2 and HCl are 434 , 242 and 431 kJ mol-1 respectively. Enthalpy of formation of HCl is :
The reaction for formation of HCl can be written as
H2+ Cl2 ⟶ 2HCI
H –H + Cl – Cl ⟶ 2 (H – Cl) Substituting the given values, we get enthalpy of formation of 2HCl = ( 676 – 862) = –186 kJ.
∴ Enthalpy of formation of HCl = \(\frac{-186}{2}\) = –93 kJ.