Attempted
Correct
UnAttempted
Wrong
Read the passage given below and answer the question that follow :J.W. Gibbs and H.Von Helmoltz had given two equations which are known as Gibbs-Helmholtz equation. One equation can be expressed in terms of change in free energy (ΔG) and enthalpy(ΔH) while other can be expressed in terms of change in internal energy (ΔE) and work function (ΔW)
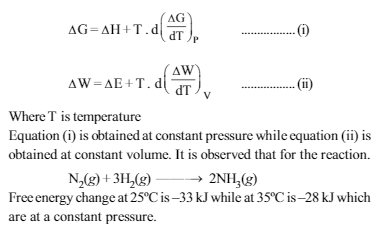
Internal energy change at 25ºC is ΔE1 while at 35ºC is ΔE2 then –
ΔE = ΔW – Td \(\left (\frac{\Delta w}{dT} \right )\)
From above relation it is clear that with increase in temperature ΔE decreases.So ΔE1 > ΔE2

Enthalpy of formation of H2O (l) is
This reaction shows the formation of H2O, and the – X2 represents the enthalpy of formation of H2O because as the definition suggests that the enthalpy of formation is the heat evolved or absorbed when one mole of substance is formed from its constituent elements.
If ΔH and ΔE are the change in enthalpy and change in internal energy respectively for a gaseous reaction, then


For a pure substance TA and TB represent the same temperature. Hence A is a correct choice.



The heat of neutralisation of HCl by NaOH is–55.9 kJ/mole.The energy of dissociation of HCN is (HCN + OH– ⟶ CN– + H2O; ΔH = -12.1 kJ)

Given that heat of neutralisation of strong acid and strong base is – 57.1 kJ. The heat produced when 0.25 mole of HCl is neutralised with 0.25 mole of NaOH in aqueous solution is :

Energy required to dissociate 4 g of gaseous hydrogen into free gaseous atoms is 208 kcal at 25°C. The bond energy of H–H will be :

The heat of neutralisation of strong base and strong acid is 57.0 kJ. The heat released when 0.5 mole of HNO3 is added to 0.20 mole of NaOH solution is
