Attempted
Correct
UnAttempted
Wrong
4HNO3 + P4O10 → 4HPO3X In the above reaction, the product X is
N2O5 is colourless deliquiscent solid. It is highly reactive, a strong oxidizing agent and is light sensitive.It is an hydride of HNO3.
Laughing gas is prepared by heating :
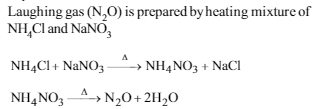
Which of the following is not oxidized by O3?
In KMnO4 manganese is already present in its highest possible oxidation state i.e. +7.So no further oxidation is possible.
Sodium thiosulphate is prepared by
\(Na_{2}SO_{3} + S \begin{matrix} medium\\ \;----\rightarrow \\ In\, alkaline\end{matrix} Na_{2}S_{2}O_{3}\)
A gas that cannot be collected over water is :
SO2 is highly soluble in water and therefore cannot be collected over water.
Sodium thiosulphate is a
Sodium thiosulphate is a reducing agent. It is used in volumetric titration(Iodimetry)to reduce I2 to I-.
\(\underset{Sod.\, thiosulphate}{2Na_{2}S_{2}O_{3}}+I_{2} \rightarrow \underset{Sod.\, tetrathionate}{Na_{2}S_{4}O_{6}}+2NaI\)
Which among the following is strongest acid?
HClO4 is the strongest acid.
Which compound is used in photography?
Hypo (Na2S2O3) is used in photography. It is used during fixing of image.It dissolves AgBr that has not been affected by light during exposure leaving metallic silver (Ag) as such
\(AgBr+\underset{Hypo}{2Na_{2}S_{2}O_{3}}\rightarrow \underset{Soluble\, complex}{Na_{3}[Ag(S_{2}O_{3})_{2}]}+NaBr\)
On electrolysis of dilute sulphuric acid using platinum electrodes, the product obtained at the anode will be
At anode : \(2OH^{-}\rightleftharpoons H_{2}O+\frac{1}{2}O_{2}\)
The oxide which form dimer is :
NO2 is the compound which forms dimer N2O4. NO2 has one unpaired electron. So,it dimerises to form paired electrons.