Attempted
Correct
UnAttempted
Wrong
During the process of electrolytic refining of copper, some metals present as impurity settle as ‘anode mud’. These are
During the process of electrolytic refining of copper Ag and Au are obtained as anode mud.
The cryolite is represented by
Na3AlF6(cryolite)is used to decrease the melting point of Al2O3 prior to electrolysis to get pure aluminium.
Froth floatation process is used for the concentration of
Froth floatation process is used for sulphide ores in which ore particles are accumulated in froth and the impurities remain in solution.
The electrolytic method of reduction is employed for the preparation of metals that

Which of the following reactions represents calcination?
Thermite process is used in reduction of
Due to high affinity for oxygen aluminium reduces several metallic oxides like Fe2O3, Cr2O3, Mn2O3 etc.
Cr2O3 + 2Al + Al2O3 + 2Cr + Heat
This process is known as thermite process.
Flux is used to :
Flux is used during metallurgy to remove silica and undesirable metal oxides.
Which of the following species has the highest electron affinity?
Fluorine is the most electronegative element. Hence its electron affinity is highest. By gaining one electron it completes its octet. Whereas oxygen needs electrons,O- is already a negatively charged species hence have some repulsion for the incoming electron. Na+, will again loose its octet by gaining electron.
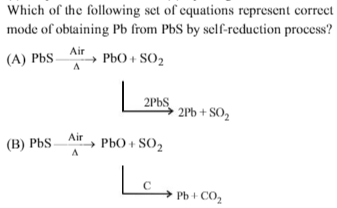
PbS reduces PbO to Pb and SO2 is liberated. This is called as self-reduction.
Process followed before reduction of carbonate ore is –
Calcination is heating ore in absence of air to remove moisture and volatile impurities.Carbonate ores decomposed to corresponding oxides as a result of calcination.