Attempted
Correct
UnAttempted
Wrong
Which one of the following is the strongest base in aqueous solution ?
Aromatic amines are less basic than aliphatic amines.Among aliphatic amines the order of basicity is 2°> 1° >3°. The electron density is decreased in 3°amine due to crowding of alkyl group over N atom which makes the approach and bonding by a proton relatively difficult. Therefore the basicity decreases. Further Phenyl group show – I effect, thus decreases the electron density on nitrogen atom and hence the basicity.
∴ dimethylamine (2° aliphatic amine) is strongest base among given choices.
∴ The correct order of basic strength is Dimethylamine > Methyl amine > Trimethyl amine > Aniline.

Which of the following statements about primary amines is‘False’ ?
Aryl amines do not produce phenol on treatment with nitrous acid.
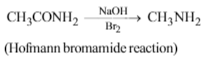
Acetamide is treated with the following reagents separately.Which one of these would yield methylamine?
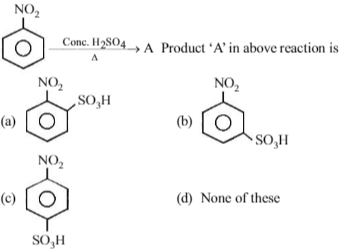
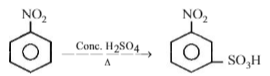
Nitrobenzene can be prepared from benzene by using a mixture of conc. HNO3 and conc. H2SO4 in the mixture, nitric acid acts as a/an :
\(HONO_{2} + H_{2}SO_{4} \rightarrow NO_{2}^{+} + H_{2}O + HSO_{4}^{-}\)
Nitric acid acts as a base by accepting a proton.
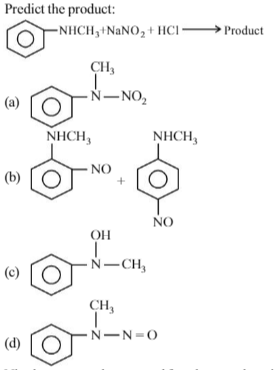
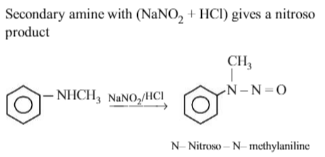
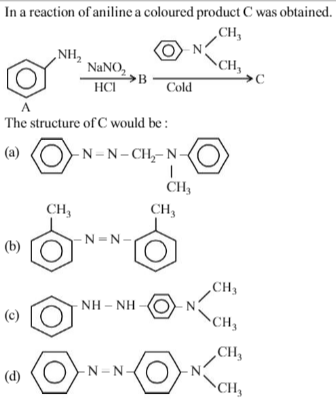
Which one of the following on reduction with lithium aluminium hydride yields as econdary amine?
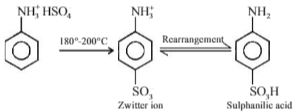
Anilinium hydrogen sulphate on heating with sulphuric acid at 453-473 K produces