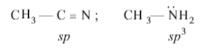Attempted
Correct
UnAttempted
Wrong
Benzamide and benzylamine can be distinguished by
Cold dil.NaOH does not attack to either of the compound,while cold dil.HCl reacts only with benzyl amine C6H5CH2NH2
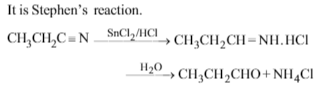
n-Propylamine yields a volatile compound X on warming with alc. alkali and chloroform. X has an offensive odour.The structure of X is
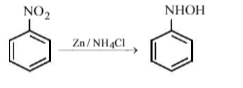
Nitrobenzene on treatment with zinc dust and aqueous ammonium chloride gives:
Hydrolysis of phenyl isocyanide forms
Hydrolysis of phenyl isocyanide forms formic acid.
When primary amine is heated with CS2 in presence of excess mercuric chloride, it gives isothiocyanate. This reaction is called

N-alkyl formamides when dehydrated with POCl3 inpresence of pyridine give isocyanides
Aromatic nitriles (ArCN) are not prepared by reaction :
Aryl halide (ArX) does not undergo nucleophilic substitution because they have strong C—X bond due to resonance.
In the reaction:
\(R – C = N \overset{x}{\rightarrow} RCH_{2}NH_{2}\)
X can be :
Among the given reagents, only LiAlH4 is the reducing agent.
Methyl cyanide is less basic than methylamine because: