What is formed, when acetonitrile is hydrolysed partially with cold concentrated HCl?
-
Solution
When acetonitrile is hydrolysed partially with cold concentrated HCl it forms acetamide.

On further hydrolysis acetamide may give either CH3COOH (acetic acid) or its salt.
Which of the following compounds would be the mainproduct of an aldol condensation of acetaldehyde and acetone?
-
Solution

Ketone upon treatment with Grignard Reagent gives
-
Solution

Hydride ion transfer takes place in
-
Solution
In the Cannizzaro reaction, two moles of carbonyl compounds having no α-hydrogen atom when treated with strong alkali undergo, redox or disproportionation reaction.

Mechanism :First of all base OH–acts as a nucleophileand attack other one of carbonyl compound to generate ahydroxy alkoxide ion which acts as a hydride iondonor to the other molecule of carbonyl compounds. In the final step there is a exchange of proton from acid to alkoxideion to get stable product.

Which of the products is formed when acetone is reacted with barium hydroxide solution?
-
Solution

Main product obtained from the reaction of ammonia and formaldehyde is
-
Solution
Formaldehyde on reaction with ammonia forms a crystalline compound, hexamethylene tetramine.

The reactant (X)in the reaction
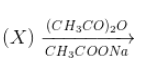
Cinnamic acid, is
-
Solution
Benzaldehyde forms cinnamic acid as follows.

The reagent which does not give acid chloride on treating with a carboxylic acid is
-
Solution
Cl2 does not give acid chloride on treating it with acarboxylic acid.
While PCl3, PCl5 and SOCl2 gives nucleophilic substitution reaction with carboxylic acid (Cl–replaces OH–group of– COOH)
Which of the following is an example of aldol condensation?
-
Solution
Aldol condensation involves an aldehyde or ketone having an α–hydrogen atom. This type of condensationoccurs in presence of dilute base (i.e., dil NaOH).Only CH3COCH3 will give aldol condensation(Both HCHO and C6 H5CHO lack α-hydrogen).
The property which distinguishes formic acid from aceticacid is
-
Solution
We can distinguish between formic acid and acetic acid by their action on Fehling’s solution. Formic acid gives ared ppt of cuprous oxide but acetic acid does not give red ppt.






