Attempted
Correct
UnAttempted
Wrong
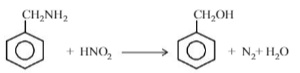
Which is formed when benzalamine react with nitrous acid
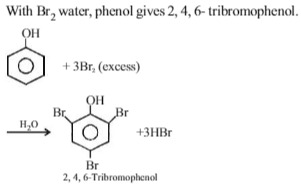
When phenol is treated with excess bromine water. It gives
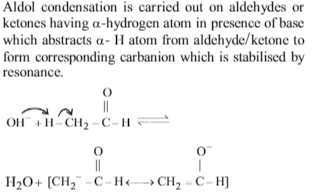
The intermediate formed in aldol condensation is
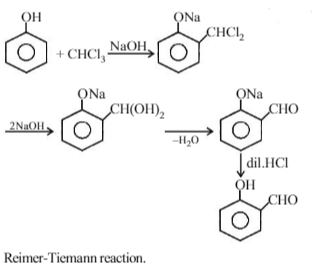
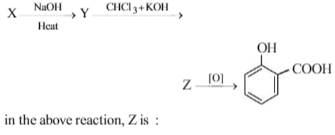
When phenol is heated with CHCl3 and NaOH then salicylaldehyde is produced. This reaction is known as
Ethanol and dimethyl ether form a pair of functional isomers.The boiling point of ethanol is higher than that of dimethyl ether, due to the presence of
Due to H–bonding boiling point of C2H5OH is much higher than isomeric (CH3)2O.
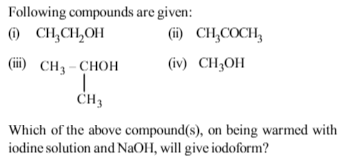
Among the given compounds only CH3OH does not give iodo form reaction.
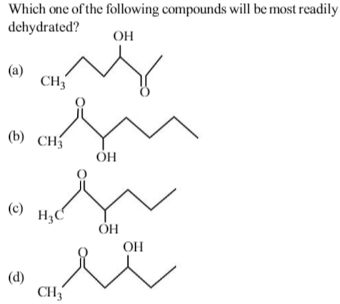
The intermediate is carbocation which is destabilised by C = O group (present on α-carbon to the –OH group) in the first three cases. In (d), α–hydrogen is more acidic which can be removed as water. Moreover,the positive charge on the intermediate carbocation is relatively away from the C= O group.
Glycerol is more viscous than ethanol due to
Because of larger (three per molecule) number of intermolecular hydrogen bonding in case of glycerol(CH2OH –CHOH – CH2OH ) as compared to ethanol(CH3CH2OH), the attraction between molecules of glycerol is more than that of molecules of ethanol. Due to this glycerol is more viscous than ethanol.