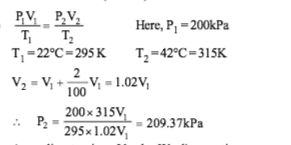A graph is plotted with PV/T on y-axis and mass of the gas along x-axis for different gases. The graph is
-
Solution

The molar heat capacities of a mixture of two gases at constant volume is 13 R/6. The ratio of number of moles of the first gas to the second is 1: 2. The respective gases may be
-
Solution

A gas mixture consists of 2 moles of oxygen and 4 moles of Argon at temperature T. Neglecting all vibrational moles, the total internal energy of the system is
-
Solution

Work done by a system under isothermal change from a volume V1 to V2 for a gas which obeys Vander Waal’s equation \((V-\beta\, n)\left ( p+\frac{\alpha n^{2}}{V} \right )\) is
-
Solution

Air is pumped into an automobile tube upto a pressure of 200 kPa in the morning when the air temperature is 22°C.During the day, temperature rises to 42°C and the tube expands by 2%. The pressure of the air in the tube at this temperature, will be approximately
-
Solution

If the potential energy of a gas molecule is U = M/r6– N/r12, M and N being positive constants, then the potential energy at equilibrium must be
-
Solution

Two monatomic ideal gases 1 and 2 of molecular masses m1 and m2 respectively are enclosed in separate containers kept at the same temperature.The ratio of the speed of sound in gas 1 to that in gas 2 is given by
-
Solution

An ideal gas is found to obey an additional law VP2 =constant. The gas is initially at temperature T and volume V.When it expands to a volume 2 V, the temperature becomes
-
Solution
Since VP2 = constant,
VP2 = 2VP'2
∴ P' = 1⁄√2
As P⁄T = constant or T α P,T=thus T becomes T/√2
N molecules, each of mass m,of gas A and 2 N molecules,each of mass 2 m, of gas B are contained in the same vessel which is maintained at a temperature T. The mean square velocity of molecules of B type is denoted by V2 and the mean square velocity of A type is denoted by V1, then V1⁄V2 is
-
Solution

Two gases A and B having the same temperature T, same pressure P and same volume V are mixed. If the mixture is at the same temperature T and occupies a volume V,the pressure of the mixture is
-
Solution