Attempted
Correct
UnAttempted
Wrong
If the molecules of a gas have f degrees of freedom, the ratio Cp/Cv of the gas is equal to :
Cp⁄Cv = 1 + 2⁄f
If the root mean square velocity of the molecules of hydrogen at NTP is 1.84 km/s. Calculate the root means quare velocity of oxygen molecule at NTP, molecular weight of hydrogen and oxygen are 2 and 32 respectively

The amount of heat energy required to raise the tempera-ture of 1g of Helium at NTP, from T1 K to T2 K is

In the given (V – T) diagram, what is the relation between pressure P1 and P2 ?

The molar specific heat at constant pressure of an ideal gas is (7/2)R. The ratio of specific heat at constant pressure to that at constant volume is

Which one the following graphs represents the behaviour of an ideal gas ?
For an ideal gas PV = constant i.e. PV doesn’t vary with V.
Figure shows a parabolic graph between T and 1/V for a mixture of a gas undergoing an adiabatic process. What is the ratio of Vrms of molecules and speed of sound in mixture?


A vessel has 6g of hydrogen at pressure P and temperature 500K. A small hole is made in it so that hydrogen leaks out.How much hydrogen leaks out if the final pressure is P/2 and temperature falls to 300 K ?

An ideal gas is taken through a process in which the pressure and the volume are changed according to the equation P =kV. Molar heat capacity of the gas for the process is

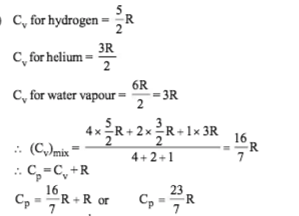
Four mole of hydrogen,two mole of helium and one mole of water vapour form an ideal gas mixture. What is the molar specific heat at constant pressure of mixture?